
FDA Issues Notices Over Unapproved Pediatric Fluoride Drugs
The U.S. FDA has announced new actions to limit the sale of unapproved ingestible fluoride

The U.S. FDA has announced new actions to limit the sale of unapproved ingestible fluoride

In recent weeks, Indian pharmaceutical manufacturer Sresan Pharma has come under public and regulatory scrutiny

What Every Pharma Executive Should Know At the 2025 FDA Generic Drugs Forum, OGD’s Darby

What’s Changing Why It Matters What Companies Should Do Now Executive Recommendation As global pharmaceutical
A new report commissioned by the U.S. pharmaceutical trade group warns that a proposed 25%
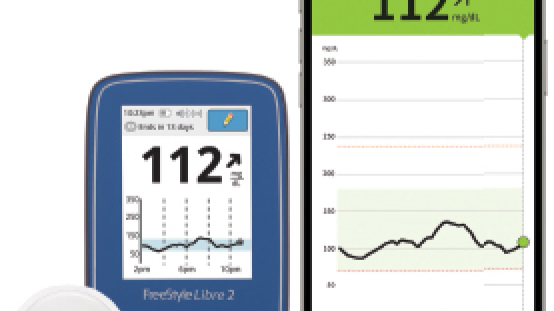
This could change how diabetes is managed across the U.S. The future of diabetes care

Leveling the Global Playing Field FDA Expands Unannounced Foreign Inspections-Are you Ready? New inspection model

A new U.S. directive puts drugmakers on the clock, will global pricing strategy shift under

The FDA has officially wrapped its first generative AI pilot for scientific review and is

The FDA has taken a significant step to address the rising cost of prescription drugs

Sign up for our monthly newsletter for the latest news & articles
Copyright ©2025 LST Consulting USA. All Rights Reserved Copyright